Are you interested in finding 'lewis structures essay'? Here, you will find all the stuff.
Jerry Lee Lewis Structure shows how atoms in A covalent bond contribution electrons. Dots ar used to appearance lone pairs of electrons, and lines symbolize bonds. This essay will excuse how Lewis Structures are constructed. The first step is counting how galore valence electrons at that place are.
Table of contents
- Lewis structures essay in 2021
- Ionic lewis structure practice
- Lewis structure how to
- Ionic lewis dot and formula practice sheet
- Lewis dot structure steps
- Lewis structures worksheet
- Valid ionic lewis structures
- Liio lewis structure
Lewis structures essay in 2021
 This image illustrates lewis structures essay.
This image illustrates lewis structures essay.
Ionic lewis structure practice
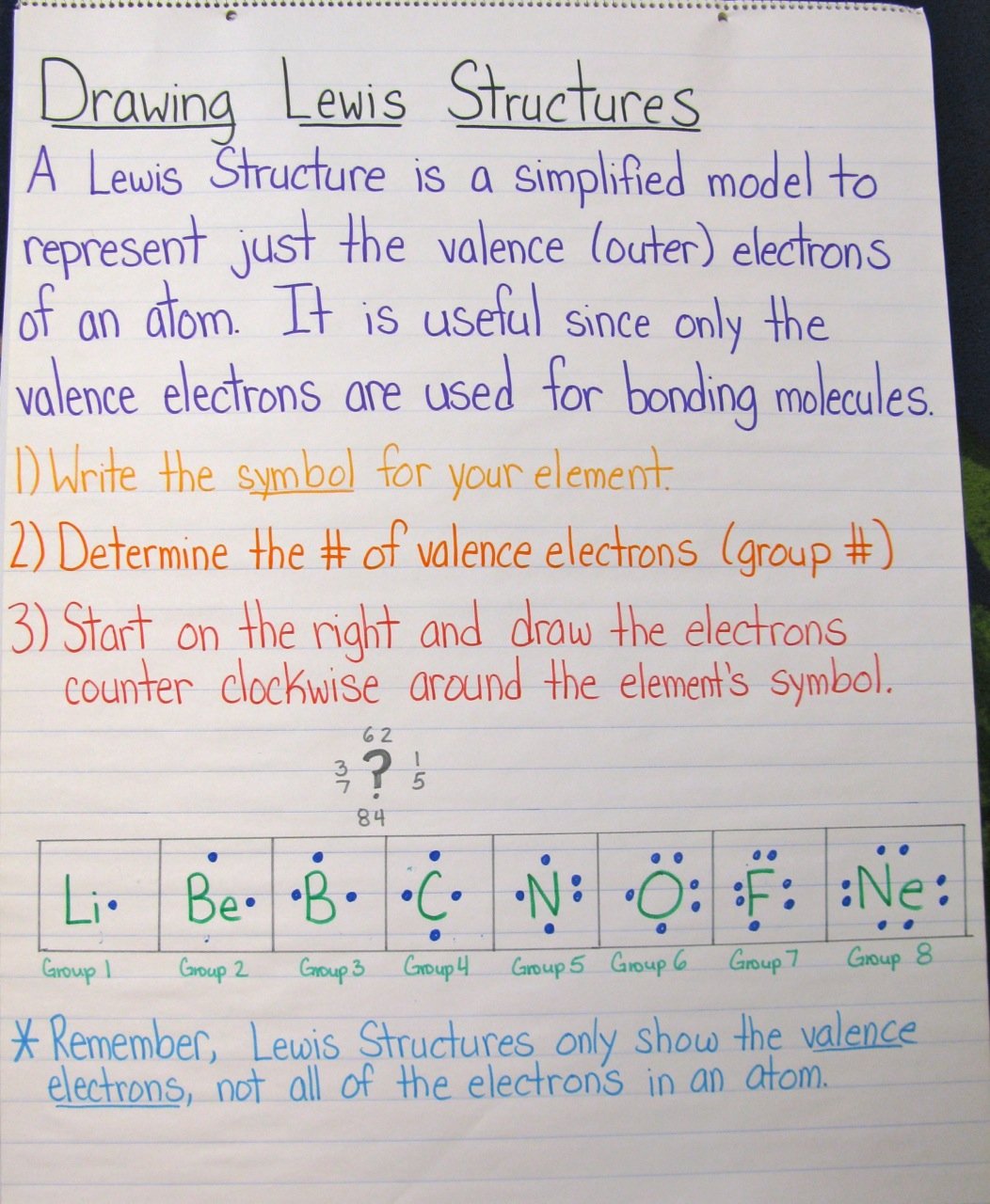 This image representes Ionic lewis structure practice.
This image representes Ionic lewis structure practice.
Lewis structure how to
 This image demonstrates Lewis structure how to.
This image demonstrates Lewis structure how to.
Ionic lewis dot and formula practice sheet
 This picture illustrates Ionic lewis dot and formula practice sheet.
This picture illustrates Ionic lewis dot and formula practice sheet.
Lewis dot structure steps
 This image representes Lewis dot structure steps.
This image representes Lewis dot structure steps.
Lewis structures worksheet
 This image shows Lewis structures worksheet.
This image shows Lewis structures worksheet.
Valid ionic lewis structures
 This image demonstrates Valid ionic lewis structures.
This image demonstrates Valid ionic lewis structures.
Liio lewis structure
 This picture representes Liio lewis structure.
This picture representes Liio lewis structure.
Why are electron dot structures called Lewis structures?
The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
How are valence electrons represented in Lewis structures?
Electron dot structure-valence electrons are represented by dots around the symbol of chemistry. Electrons sharing — covalent bonding — electrons sharing is the glue that holds atoms together. What is the purpose of Lewis structures?
Why is the Lewis structure important in chemistry?
For the prediction of geometry, polarity and reactivity of (in)organic compounds, Lewis structures are actually very important. The Lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom.
Last Update: Oct 2021